Recombinant Erwinia Asparaginase Production
Background
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children. Lymphoblastic lymphoma (LBL) is a rare, fast-growing, aggressive subtype of non-Hodgkin’s lymphoma most often seen in teenagers and young adults. Asparaginases are a key component of a multi-agent chemotherapeutic regimen for the treatment of ALL and LBL.¹
Challenge
Due to worldwide supply challenges, patients with hypersensitivity to an E. coli-derived asparaginase had a critical need for an immunologically distinct asparaginase for their prescribed treatment plan. The Pfenex Expression Technology® was used to solve this problem in less than six years.
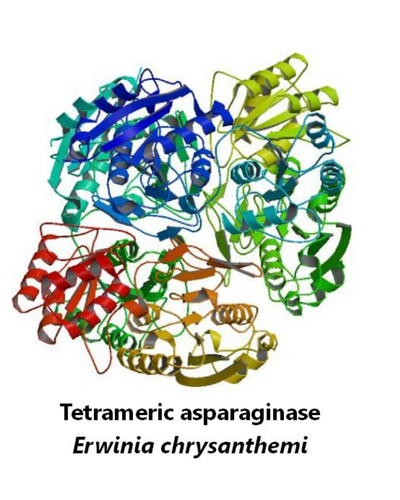
Solution
- High-titer expression strains identified at 96 well scale
- Over 20g/L with 2L baseline process
- Preclinical study material produced in three months
- Process development in eight months
- Developed process transferred to GMP
Key Results
- Chromosomal KOs of native L-asparaginase of P. fluorescens host strains developed
- Five strains scaled to 2L for evaluation under multiple induction conditions
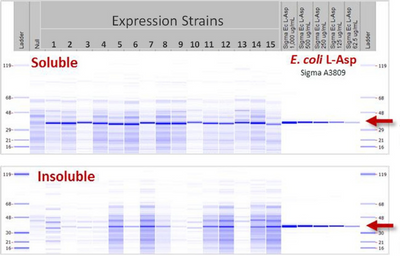
Strain 4 cultivated under 8 induction conditions
Screening factors: wet cell weight at induction,
IPTG concentration, pH, temperature
TAKEAWAYS
- Project kick-off to FDA approval in ~5 yrs
- Compressed program timeline: CMC development key to critical path
- Phase 3 ready process enabled smooth transfer and process characterization
- Solved a critical industry and patient need
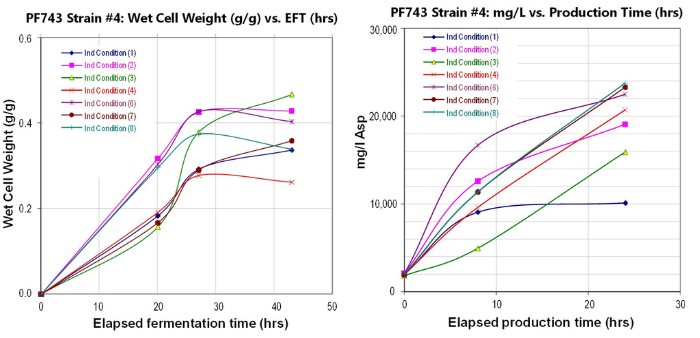
Fermentation paste from 2L scale
used to support preclinical testing
The close collaboration between the Primrose and Jazz teams resulted in the effective launch of a critical oncology treatment via the Pfenex Expression Technology® platform.